In a new study from LVPEI, Naheed A. Borah, Dr. Mamatha M. Reddy, and others describe how aurora kinase A, a cell cycle regulator protein, plays a key role in the poor prognosis and chemotherapy resistance of retinoblastoma, and is a potential therapeutic target.
Retinoblastoma is an eye cancer affecting children aged five years or younger. In retinoblastoma, a malignant tumor develops in the retina, the inner layer of the eye. The cancer can spread, becoming fatal, unless treated early. The prognosis for retinoblastoma in South Asia is poor. This cancer is usually caused by mutations in RB1, a tumor suppressor gene that controls cell division in healthy retinal cells. In rare cases, dysregulation of MYCN, an oncogene essential for fetal organ development, can also manifest as retinoblastoma despite having a functional RB1. Usually, chemotherapy is used to treat retinoblastoma, which can lead to side-effects, systemic toxicity, and treatment resistance (chemoresistance). Molecular therapies, which rely on inhibiting specific genes or proteins, hold the promise of a better treatment for retinoblastoma. But before such next-generation therapeutics can be designed, it is necessary to identify molecular targets.
Aurora kinase A (AURKA) is a key cell cycle regulator protein that controls mitotic cell division. Research shows that AURKA inhibition in lung cancer cells lacking active RB1 leads to their cell death. AURKA has also been shown to prevent MYCN protein degradation in neuroblastoma, promoting cancer cell proliferation. Since retinoblastoma is driven by alterations in RB1 and MYCN, could AURKA play a crucial role in driving its progression? If so, could this protein be a viable target for molecular therapy?
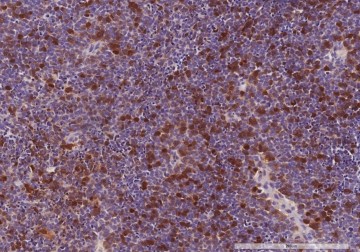
In a new study published in The American Journal of Pathology, Naheed A. Borah, Dr. Mamatha M. Reddy, and others from LVPEI investigate the role of AURKA in retinoblastoma. The study included tumor tissue samples from 67 patients. Immunohistochemical analysis revealed that AURKA is overexpressed in retinoblastoma. More than 73% of the samples showed increased expression in the tumor, but no expression in the adjacent healthy retinal tissue. Increased expression also correlated with one or more histopathologic high-risk factors such as the tumor spreading to other parts of the eye, including the optic nerve and the sclera (the white of the eyes).
To see what happens to retinoblastoma cells in the absence of AURKA, the researchers ‘silenced’ AURKA expression in two retinoblastoma cell lines using small hairpin RNAs. Reduced expression of AURKA led to increased cancer cell death. Pharmacological inhibition of AURKA with small molecule inhibitors exacerbated tumor cell death over 72 hours. This indicates that elevated AURKA levels promote retinoblastoma cell survival. The study also noted that AURKA and MYCN crosstalk, creating a positive feedback loop to maintain the malignant state of the tumor. These findings raise the possibility that targeted AURKA inhibition could be a therapeutic avenue for managing retinoblastoma.
‘Targeted inhibition of AURKA in retinoblastoma can induce tumor cell death and thus holds therapeutic potential. This finding aligns with our previous study, where we discussed another potential anti-cancer target, Aurora kinase B (AURKB),’ concludes Dr. Mamatha M. Reddy, research scientist at LVPEI and the corresponding author of this paper. ‘But before they can be implemented as treatments it is necessary to develop better approaches towards targeted protein inhibition—with high specificity and reduced toxicity.’
Citation
Borah, N. A., Mittal, R., Sucharita, S., Rath, S., Kaliki, S., Patnaik, S., Tripathy, D., & Reddy, M. M. (2024). Aurora Kinase A Is Overexpressed in Human Retinoblastoma and Correlates with Histopathologic High-Risk Factors: Implications for Targeted Therapy. The American Journal of Pathology, S0002-9440(24)00205-0. Advance online publication. https://doi.org/10.1016/j.ajpath.2024.05.006
Photo credit: Close-up of AURKA protein expression, Fig. 1, Naheed et al.