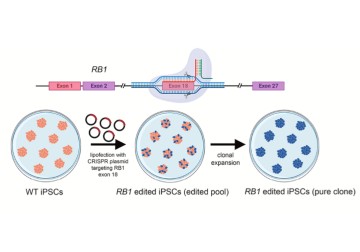
In a new study from LVPEI, Trupti Agrawal, Savitri Maddileti, and Dr. Indumathi Mariappan have generated three human induced pluripotent stem cell lines lacking the RB1 gene, using the CRISPR/Cas9 system. These genetically modified stem cells can be used as in vitro models for retinoblastoma research.
Retinoblastoma is an ocular cancer which begins in the retina, the innermost layer of the eye. This cancer affects children, often two years or younger, and can be sight- or even life-threatening unless diagnosed and treated in its initial stages—a tall order in low- and middle-income nations. Mutations in the RB1 gene, a tumor suppressor which regulates DNA replication and cell division in healthy cells, leads to Retinoblastoma. Understanding the pathophysiology of retinoblastoma and the role of RB1 is crucial for developing new modes of diagnosis and treatment. A variety of constraints, including lack of access to early-stage tumor tissues, make it difficult to understand the exact mechanisms leading to full blown cancers.
Induced pluripotent stem cells (iPSCs) can generate most cell types of the body for research and therapeutic needs. iPSCs are stem cells that have been biochemically reprogrammed to attain ‘pluripotency,’ the ability to self-renew and differentiate into any kind of cell. iPSCs generated from skin/blood cells can be chemically triggered to become retinal cells. If the RB1 gene can be knocked off in such reprogrammed iPSCs without affecting their pluripotency, then such cell-lines are of immense value for retinoblastoma research. The CRISPR/Cas9 system is a revolutionary gene editing technology that can introduce precise changes at desired target sites in genomes. Such systems with reduced off-targets enable the generation of gene knock out models for research.
In a new ‘lab resource’ paper published in Stem Cell Research, Trupti Agrawal, Savitri Maddileti, and Dr. Indumathi Mariappan, demonstrates the success of CRISPR/Cas9 technology in generating three human iPSC (hiPSC) lines lacking a functional RB1 gene, mirroring mutant retinal cells in retinoblastoma. The complete loss of RB1 was achieved by editing exon 18 (an exon is a sequence of DNA that gets copied in the mRNA and codes for the protein molecule) of the RB1 gene in an iPSC line derived from the umbilical cord blood of a healthy volunteer. Using an engineered guide RNA (gRNA), the team used Cas9, a DNA slicing protein, to create random base insertions/deletions, creating frameshift mutations precisely in the RB1 gene, which results in a truncated and non-functional protein.
One of these iPSC lines carried a homozygous mutation (both copies of the RB1 gene were disrupted with identical mutations), while two lines had compound heterozygous mutations (both copies were disrupted, but by different mutations). These cell lines with non-functional RB1 were clonally expanded—proliferated via rapid cell division while keeping every cell a clone of the parent iPSC—to generate pure lines and were genotyped to confirm the mutations and genomic integrity. The mutant iPSC lines retained their stemness properties and the ability to generate multilineage cell types of the body.
‘These RB1 null iPSC lines can be used to generate retinal organoids, which are like miniature forms of fetal stage retina. Since retinoblastoma arises during early retinal development, these stem cell models allow us to evaluate how loss of RB1 alters normal retinal tissue formation’, says Ms. Trupti Agrawal, a Senior Research Fellow, and the lead author in the study.
‘Excised tumor tissue samples from patients are valuable to understand the status of late-stage cancers, their response to drugs and disease prognosis. However, we know little about the cell-of-origin and early molecular insults that trigger such cancerous transformations,’ explains Dr. Indumathi Mariappan, a research scientist at the Sudhakar and Sreekanth Ravi Stem Cell Biology Laboratory, LVPEI and the corresponding author of this paper. ‘This requires alternate methods to evaluate cancers at early stages. This knowledge gap can be effectively addressed using human-relevant and in vitro disease models such as the RB1 null iPSC lines reported in this study. Such mechanistic understanding can help us to identify precise targets and to develop improved drugs for combating the disease at initial stages.’
Citation
Agrawal, T., Maddileti, S., & Mariappan, I. (2024). Generation and characterization of three CRISPR/Cas9 edited RB1 null hiPSC lines for retinoblastoma disease modelling. Stem cell research, 76, 103373. https://doi.org/10.1016/j.scr.2024.103373
Photo credit: Fig 1, Agrawal et al.