In a new study from LVPEI, Madhuri Amulya Koduri, Dr. Swapna S. Shanbhag, Dr. Sayan Basu, Dr. Vivek Singh, and others analyzed inflammatory proteins and cytokines in human tears that play a role in ocular complications following Stevens–Johnson syndrome.
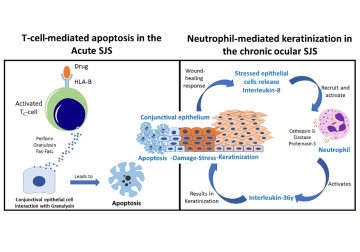
Stevens–Johnson syndrome (SJS) and its severe variant, toxic epidermal necrolysis (TEN), are drug-induced severe allergic reactions in the skin and mucous membranes to medication. People with SJS/TEN develop rashes, ulcers, and blisters on mucous membranes, a moist layer of epithelial cells that lines our internal organs and cavities, including our eyes. SJS in the eyes affects the conjunctiva, a mucous membrane that lines the eyelid and ocular surface, making the interior eyelid rough and keratinized—hard tissue debris that makes the membrane scaly and dry. Without immediate treatment, friction from keratinized eyelids erodes the ocular surface (cornea) and depletes limbal stem cells, leading to blindness.
At the molecular level, a flood of cytokines, proteins that regulate inflammation in tissues, and other inflammatory proteins are responsible for much of the tissue damage in SJS. These proteins accumulate in tears, where they inflame lacrimal glands and sebaceous (Meibomian) glands involved in producing the tear film. Knowing which proteins are involved in SJS and how they are involved will not only provide a deeper understanding of the disease, but the proteins could also be used as biomarkers for diagnosing SJS at an early stage. Since SJS and TEN are rare diseases, and thus the molecular mechanisms involved in recruiting these proteins remain unclear. Moreover, hundreds of cytokines and many more proteins are produced in the eye. The specific ones involved in ocular complications in SJS are difficult to find unless all proteins found in tears (the tear proteome) are analyzed.
In a new study published in the journal Allergy, Madhuri Amulya Koduri, Dr. Swapna S. Shanbhag, Dr. Sayan Basu, Dr. Vivek Singh, and others from LVPEI did just that. The team analyzed all the proteins and cytokines in human tears to identify those that play a role in ocular complications following SJS/TEN. Tear samples were taken from 40 patients with SJS/TEN and compared with an equal number of age-matched healthy controls. Tear fluid proteins from 11 patients were quantified using mass spectrometry (MS). The identified proteins were then validated in all patients using ELISA, a biochemical assay that detects specific proteins. The team also analyzed eyelid margin tissue samples from patients using immunohistochemistry.
Using MS, 1,692 tear fluid proteins were identified, among which 470 proteins were either upregulated or downregulated significantly (log 10-fold change) in SJS/TEN patients. The most upregulated proteins in tears included neutrophil elastase, defensin, and matrix metalloproteinase, while myeloperoxidase was upregulated in eyelid margins. All these proteins are typically secreted by neutrophils, a type of white blood cell and a key part of human immune response, indicating that tissue inflammation in SJS is likely driven by neutrophils. Among the 1,692 proteins, 41 were only found in SJS/TEN patients. IL-36γ, a cytokine that promotes neutrophil-driven inflammation, was one of those unique proteins. IL-36γ, along with other unique proteins, has the potential to act as biomarkers for eyelid margin keratinization in SJS. Finally, most of the downregulated proteins were lacrimal gland secretions, indicating dysregulation of tear production in SJS/TEN.
‘Our primary line of prevention against Stevens-Johnson syndrome (SJS) is to consider genetic testing for the HLA-B*1502 gene variation before administering certain medications,’ explains Dr. Vivek Singh, principal scientist at the Sudhakar and Sreekanth Ravi Stem Cell Biology Laboratory and the corresponding author of this paper. ‘SJS, often triggered by specific drugs, typically manifests within 1-3 weeks of starting the medication. Managing this complex disorder is challenging, and our team is actively researching the roles of IL-36 and neutrophils in the disease’s pathogenesis to gain deeper insights and finally creating animal models to validate it.’
Citation
Koduri, M. A., Pingali, T., Prasad, D., Singh, V., Singh, S., Shanbhag, S. S., Basu, S., & Singh, V. (2024). Neutrophil-driven and interleukin-36γ-associated ocular surface inflammation in chronic Stevens-Johnson syndrome. Allergy. Advance online publication. https://doi.org/10.1111/all.16126
Photo credit: Fig 9, Koduri et al.