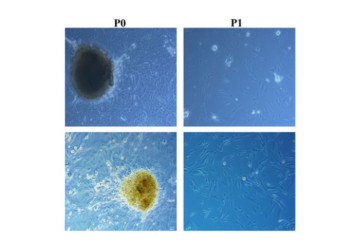
A new paper by Drs Sayan Basu, Vivek Singh, and others from L V Prasad Eye Institute studies the ability of an ethically sourced, serum free, chemically defined, proprietary medium to support the harvest and re-seeding of human limbal mesenchymal stem cells for a few generations, for therapeutic use.
Mesenchymal stem cells (MSCs) are multipotent adult stem cells that can differentiate into various connective tissue across the body. In the human eye, the limbus, on the cornea-sclera junction, is home to corneal stromal stem cells (CSSCs). These limbal stem cells develop into keratocytes, connective tissue cells, which help repair the cornea when there is injury or infection. Any damage to the stroma though can result in these keratocytes stitching together to form scars on the clear corneal tissue, impairing vision. The only solution to such scarring is a corneal transplant, with all its attendant disadvantages including immune rejection. A new set of therapeutics, like stem cell therapies, are focused on finding alternatives that do not have these disadvantages.
For stem cell therapy to work, it is necessary that the target stem cells hold their specific phenotype and differentiate to relevant cell types over induced generations. The lab-grown cells must be colony-forming and be functional in their biochemical environment. In the case of stromal MSCs, they must sustain their spindle-like shape and express a specific set of cell surface markers. To achieve this, MSCs are grown in a lab supported on a growth medium that aids the cells’ attachment, growth, and proliferation. The predominantly used growth medium today is dependent on a foetal bovine serum (FBS) supplement. FBS is a serum cocktail derived from a bovine foetus, with thousands of uncharacterised proteins and metabolites. Every aspect of this supplement is problematic: the ethics of animal cruelty, its sourcing and distribution, and the huge variation in FBS composition. The stem cell therapy story then is inextricably linked to finding a neutral and viable growth medium.
In a new paper in Stem Cell Research Therapy, Drs Vivek Singh, Sayan Basu, Abhishek Sahoo, and others compared the cell viability, marker expression, differentiation, migration, and other characteristics of human limbal mesenchymal stem cells (hLMSCs) in foetal bovine serum supplemented medium, and a proprietary Serum-free medium (SM): StemMACS MSC Expansion Media. The study checked the cells for their ‘wound healing’ and colony-forming ability over time. The samples’ gene expression was analysed with a real-time PCR using markers and a cadaveric limbal sample as control.
The study found that the serum-free medium was suitable for the hLMSC cultures, and in some instances, enhanced their characteristics when compared to FBS fortified medium. SM showed more cumulative—and stable--population doubling time and more viability than the medium with FBS. It noted no significant difference in the phenotypic expression and both media successfully aided adipogenic, chondrogenic, and osteogenic differentiation. While cultures in both media healed wounds, a well-known characteristic of MSCs, SM cultures had better potential and had better colony-forming abilities too. In all, this study makes a strong case for replacing FBS with a serum-free medium, many of which are now available in the market.
'Our study demonstrated for the first time, the feasibility of using animal cell-free media for growth of limbal stem cells to very late passages, i.e., more cultures leading to more cells, without altering its properties,' says Dr Vivek Singh, Scientist, Sudhakar and Sreekanth Ravi Stem Cell Biology Laboratory and one of the authors of this study.
'It also helps us to have more regulated performance along with increased productivity or harvesting more cells as well as cell-based products like exosomes and secretomes which are clinically important for treating many diseases. This work also provides a template that can be replicated by various groups across the world working in limbal stem cell/cell-based therapy to transition to serum-free media.'
'The corneal stem-cell research team led by Basu-Singh has recently achieved a milestone by successfully completing two pioneering clinical trials that demonstrate the safety and efficacy of GMP-manufactured hLMSCs. These trials utilized hLMSCs to treat challenging ocular conditions like corneal scarring, ulcers, and burns - a first in the realm of eye care,' says Dr Sayan Basu, the Prof D. Balasubramanian Chair of Eye Research at LVPEI, and one of the authors of this paper.
'As we build on this success, the team is committed to using serum-free and xeno-free products in our therapeutic and regenerative medical research.'
Citation
Sahoo, A., Damala, M., Jaffet, J. et al. Expansion and characterization of human limbus-derived
stromal/mesenchymal stem cells in xeno-free medium for therapeutic applications. Stem Cell Res Ther 14, 89 (2023).
https://doi.org/10.1186/s13287-023-03299-3
Photo credit: Truncated micrograph of hLMSCs cultured in SM & Control medium; Sahoo et al.